







 |
 |
|
    

|
||


When a researcher has a long list of genes to analyze, as is frequently the case with the results from a library or gene chip experiment, the time and effort involved in examining the literature can be significant. StAT allows the researcher to quickly search and analyze the literature concerning a gene in an effective manner. For example, consider the protein Zinc-alpha-2-glycoprotein (ZAG) for which MEDLINE has 59 abstracts. The researcher could examine these abstracts individually, a time consuming and tedious task. StAT speeds up the review process by quickly identifying a ranked list of the key sentences and most important abstracts for the user.
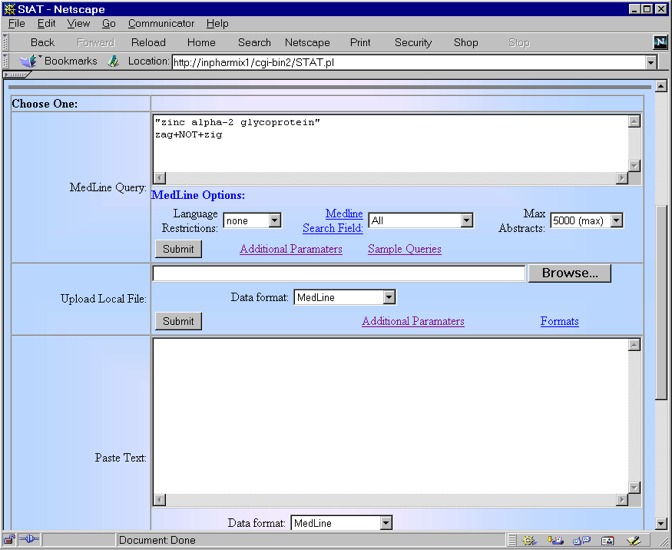
The query above is parsed by the StAT processor and passed to MEDLINE as;
"zinc alpha-2 glycoprotein" OR (zag NOT zig)
In our ZAG example, the researcher learns that ZAG is a ubiquitous serum protein, that it has a non-peptidic MHC like binding groove and that an X-ray crystal structure is available, all without reviewing the entire body of publications.
| Score | Link to Marked Abstract | Key Sentence |
|---|---|---|
| 4355 272 |
0010376801 | Zinc-alpha2-glycoprotein (Znalpha2gp) is a soluble major histocompatibility complex homolog widespread in body fluids and in glandular epithelia; the authors recently demonstrated its presence in stratified epithelia. |
| 4252 354 |
0009328826 |
Zinc-alpha 2-glycoprotein (Zn alpha 2gp) is almost ubiquitous in body fluids, and its antibody labels the corresponding secretory epithelia. |
| 4250 250 |
0010206894 | The 2.8 angstrom crystal structure of ZAG resembles a class I major histocompatibility complex (MHC) heavy chain, but ZAG does not bind the class I light chain beta2-microglobulin. |
>0010206894 Crystal structure of human ZAG, a fat-depleting factor related to MHC molecules. Zn-alpha2-glycoprotein (ZAG) is a soluble protein that is present in serum and other body fluids. ZAG stimulates lipid degradation in adipocytes and causes the extensive fat losses associated with some advanced cancers. The 2.8 angstrom crystal structure of ZAG resembles a class I major histocompatibility complex (MHC) heavy chain, but ZAG does not bind the class I light chain beta2-microglobulin. The ZAG structure includes a large groove analogous to class I MHC peptide binding grooves. Instead of a peptide, the ZAG groove contains a non-peptidic compound that may be implicated in lipid catabolism under normal or pathological conditions.
The StAT results include links back to the original MEDLINE records. In just a few minutes, the researcher can make an informed decision on the merits of a given gene for further research.