So, you've glued together some hunks of PVC, installed an ignition system and bought a bag of russets. What should you use for fuel to get the most oomph into that hunk of starch? There are many ways to judge the best fuel for a combustion spud gun. How easy is it to use? How expensive is it? And, perhaps most important to the average spudder, which fuel will launch the spud at the highest speed?
In order for combustion to occur you need two things, fuel and oxygen. In a typical spudgun, air is the source of the oxygen and the fuel is a gaseous hydrocarbon such as propane. To get the maximum amount of power out of spudgun the ratio of fuel to oxygen must be correct.
The complete combustion of hydrocarbon fuels using oxygen (O2) produces carbon dioxide (CO2), water and heat. The balanced chemical equation for the complete combustion of propane (C3H8) in oxygen is;
C3H8 + 5O2 = 3CO2 + 4H20 + Heat
The equation above tells us that for each molecule of propane we need five molecules of oxygen. If we have too much or too little fuel, the reaction does not go to completion and the energy released by the combustion is decreased.
For every fuel the maximum energy will be produced if the ratio of fuel to oxygen is correct. However, most spudguns use air as the oxygen source instead of pure oxygen. Air is about 21% oxygen (by volume) and 88% nitrogen and about 1% other gases. We have to take this into account when calculating the amount of fuel to use in a gun. The chemical equation for the combustion of propane indicates that we need 5 volumes of oxygen per volume of propane. Since air is 21% oxygen then we need about (1/5*21%)=4% by volume propane in the chamber with the air.
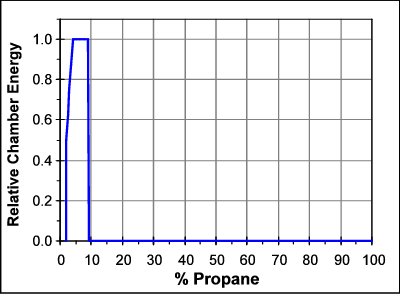
Most fuels only burn over a fairly narrow range of concentrations in air. For propane, that range is about 2.5% to 9.5% propane. If we have less than 2.5% propane, or more than 9.5%, the propane will not burn. The graph below shows the energy released for the combustion of propane in air as a function of the percentage of propane in the chamber.
So, too much fuel, or too little fuel, will simply not ignite. The optimal amount of fuel is what is predicted by the combustion equation and the percentage of oxygen in air. Too much fuel will never cause a spudgun to fail since with too much fuel the gun won't even fire.
The modern history of spudguns goes back at least fifty years.
The
basic concept of a "spudgun" is much older than that.
Alessandro Volta (as in Volt)
built what may be called the first electrically fired combustion
"spudgun" in the 1780's;
The
"electric-phlogopneumatic" pistol
was thought up by Volta. It was generally filled with a mixture of
hydrogen and air, and then corked up. One of the electrodes
was touched with one hand; the other hand touched one of the poles of
an electrostatic machine. When the spark
went off between them and also inside the pistol, a loud explosion
resulted which violently shot out the cork. As
Volta himself wrote, these experiments "stupefied the ordinary
observer… caused considerable satisfaction among amateurs and those in
the know, for these are experiments combining
electricity and inflammable air…". They responded to the double
requirement, which was popular at the time, of "being showy" and of
popularizing the latest scientific results. (From here.)
Apparently, even eighteenth century scientists enjoyed launching random things, with accompanying noise, for the shear joy of doing it. (Volta's Pistol, Another site).
Volta used hydrogen as his fuel. More recent spudguns use gaseous hydrocarbons such as butane or propane. Early modern spudguns used cheap, and readily available, fuels.
RightGuard, one of the traditional spudgun fuels, has undergone various changes in composition over the years. Some types of RightGuard are still useable as a spudgun fuel. If the label has a warning about flammability and/or the ingredient lists includes things like butane, isobutane, or propane then it is usable as a fuel. There is relatively little difference between these three ingredients. Even the optimum volume percents are fairly close to each other. Since RightGuard is generally used in the "squirt and screw" fueling mode, the actual percent of fuel is difficult to control. It seems likely that the other ingredients in the can make up a fairly low percentage and probably have a relatively minor affect on the energy of the fuel.
Aquanet, and similar hair sprays, have many of the same characteristics as RightGuard. If the label warns that it is flammable, and if the ingredient includes things like butane, isobutane, or propane then it is probably usable. As with RightGuard, the other ingredients in the can, which may or may not be flammable, probably constitute a fairly low percentage of the gas. There is the possibility that the other ingredients may gum up the spark gap, fan or cleanout plug threads after several uses.
By definition, automotive starter fluid is combustible. There are no doubt a couple of different formulations, but one common formulation includes 10~30% (weight) dimethyl ether and a propane propellant. Most formulations should behave about the same as pure propane.
Pretty much any combustible compound that evaporates significantly at ~70F can be used as fuel in a combustion spudgun. Methanol, ethanol, isopropanol (rubbing alcohol), gasoline, acetone (nail polish remover), paint thinner etc. can all be used as fuel. The challenge with these liquid fuels is getting them to evaporate in the gun in a reproducible fashion. The actual energy content of this type of fuel is fairly irrelevant since the shot to shot reproducibility is so poor. It really doesn't matter, with this type of fuel, whether the predicted muzzle velocity is 10% great with one fuel versus another since the shot to shot variability is probably more like 50%. (Even with precisely metered propane, the shot to shot variability in muzzle velocity for shooting spuds is typically in the 10 to 20% range.)
One thing that you should keep in mind with liquid fuels is the potential for weakening the PVC. Take a look at the ingredients list on your cans of PVC cleaner and glue. Anything listed on those cans should probably be avoided as fuels. Acetone and tetrahydrofuran (THF) in particular are probably not the best idea for fuel since they soften PVC.
Most advanced spudders no longer use the "squirt and screw" fueling method traditionally used for RightGuard and hair spray. There is just to much variability in the performance of the gun using that method. Instead, a well defined fuel, such as pure propane, is measured accurately to fuel a spudgun. With a defined fuel, and accurate measurements, it becomes possible to consider which fuel(s) will give the most "bang for the buck".
The "heat of combustion" of a fuel is a measure of the amount of energy released when the fuel is burned. This is an important, but not the only, factor affecting the performance of a fuel. The table below lists the heats of combustion along with other parameters for a variety of pure fuels.
Heats of combustion can be measured in several different ways and this makes it difficult to find a consistent set of values for various fuels. Web based sources list both "high heat" values (which are obtained assuming the water produced condenses to liquid) and "low heat" values (which assume the water is present as steam) as well as thermodynamic heats of combustion. For this reason, the table below lists multiple values for most of the fuels. To obtain a consistent set of values for comparison purposes, I have used the red ones which were calculated using this method.
The "Displasive Volume Percent" column gives the stoichiometric volume of fuel required if the fuel displaces some of the air in the chamber when it is injected, for example when fueling with the "squirt and screw" method or using a syringe. The "Additive Volume Percent" gives the amount of fuel required when the fuel does not displace air from the chamber, for example when using a pressurized meter system.
The key value for comparing two fuels based on their heats of combustion is not the actual heats of combustion. Instead, the "Heat per mole Oxygen" should be used since the amount of energy in the combustion chamber is limited by the amount of oxygen present in the chamber. Fuel is added to match that amount of oxygen. As you can see from the table, there is relatively little difference between the various fuels based on their "Heat per mole Oxygen" values. The only two fuels with significantly higher values are hydrogen and acetylene, both of which give 10~15% more energy than the other fuels.
| Fuel | Heat
of Combust. (Kcal/mole) |
Combustion
Limits in Air (Vol%) |
Boiling
Point, C (F) |
Mole
O2 per mole Fuel |
Heat
per mole Oxygen (Kcal) |
Displasive Volume Percent(1) |
Additive Volume Percent(1) |
Molecular
Weight (g/mol) |
Combustion Equation |
| Hydrogen | 68.3 62.5 59.7 |
4.0 - 74.2 | -253 (-423) |
0.5 | 119 | 29.5 | 41.9 | 2 | 2H2 + O2 = 2H20 |
| Methane | 192 191 212 215 |
5 - 15 | -162 (-259) |
2 | 108 | 9.48 | 10.5 | 15 | CH4 + 2O2 = CO2 + 2H20 |
| Ethane | 373 340 371 |
3 - 12.5 | -89 (-184) |
3.5 | 106 | 5.65 | 5.99 | 30 | 2C2H6 + 7O2 = 4CO2 + 6H20 |
| Ethylene (Ethene) |
315 337 337 |
2.8 - 28.6 | -103.7 (-155) |
3 | 112 | 6.53 | 6.98 | 28 | C2H4 + 3O2 = 2CO2 + 2H20 |
| Acetylene | 322 301 |
2.5 - 80 | -81 (-119) |
2.5 | 120 | 7.73 | 8.38 | 26 | 2C2H2 + 5O2 = 4CO2 + 2H20 |
| Propane | 525 487 530 527 |
2.37 - 9.5 | -42.1 (-44) |
5 | 105 | 4.02 | 4.19 | 44 | C3H8 + 5O2 = 3CO2 + 4H20 |
| Butane | 684 633 687 683 |
1.86 - 8.41 | -0.5 (31) |
6.5 | 105 | 3.12 | 3.22 | 58 | 2C4H10 + 13O2 = 8CO2 + 10H20 |
| iso-Butane | 683 | 1.86 - 8.41 | -11.7 (11) |
6.5 | 105 | 3.12 | 3.22 | 58 | 2C4H10 + 13O2 = 8CO2 + 10H20 |
| MAPP (2) | |||||||||
| Methyl Acetylene | -23.2 (-9.8) |
4 | 4.98 | 5.24 | 40 | C3H4 + 4O2 = 3CO2 + 2H20 | |||
| Propadiene | 457 | -34.5 (-30) |
4 | 114 | 4.98 | 5.24 | 40 | C3H4 + 4O2 = 3CO2 + 2H20 | |
| Propylene(3) (propene, MAP/Pro) |
491 | ? | -47.6 (-54) |
4.5 | 109 | 4.7 | 4.9 | 42 | 2C3H6 + 9O2 = 6CO2 + 6H2O |
| Diethyl Ether |
647 | 1.85 - 36.5 | 34.5 (94) |
6 | 108 | 3.37 | 3.49 | 74 | C4H10O + 6O2 = 4CO2 + 5H20 |
| Dimethyl Ether |
329 |
3.0 - 18.6 |
-25 (-13) |
3 |
110 | 6.53 | 6.98 | 46 |
C2H6O + 3O2 = 2CO2 + 3H20 |
http://members.nuvox.net/F~on.jwclymer/Frq/Fhoctable.html
http://home.fuse.net/clymer/rq
combustion calculator
http://www.nrc.gov/freading-rm/fdoc-collections/fnuregs/fstaff/fsr1805/ffinal-report/f15_explosion_calculations.xls
The heat of combustion is not the only factor affecting the power of a particular fuel. The rate at which the fuel burns and the maximum temperature and pressure obtained from the fuel also affects the performance of the gun.
Gaseous fuels burn at widely varying rates. For a combustion spudgun, the faster the fuel burns the better the gun will perform. Unfortunately, there is not a lot of information available on the burn rates of various fuels. The table below lists a few fuels of interests (values from Hovedoppgave.pdf). The flame front speed is how fast the flame moves through the mixture at ambient conditions. In an actual combustion spudgun the flame front accelerates as the temperature in the chamber rises. The flame front speed as a function of temperature and pressure can be estimated using;
Flame speedi = (Flame speed0)*(Ti/T0)alpha*(Pi/P0)beta
Where Flame speed0, alpha and beta are the values shown in the table below and Flame speedi is the speed at temperature Ti and pressure Pi.
| Fuel | Flame
Front Speed meters/sec (FPS) |
Maximum Explosion Pressure (ATM) |
Adiabatic
Flame Temperature, C (F) |
alpha | beta |
| Methane |
0.45 |
8.6 |
2591 (4696) |
2.0 |
-1.5 |
| Propane |
0.43 |
9.2 |
2633 (4771) |
2.13 |
-0.17 |
| Hydrogen |
3.25 |
7.9 |
2755 (4991) |
1.26 |
0.26 |
| Acetylene |
1.55 |
9.6 |
2918 (5284) |
2.0 |
-0.06 |
As the table above shows, simple hydrocarbons such as methane and propane, behave similarly, with relatively slow flame front speeds and similar peak pressures and temperatures. Hydrogen and acetylene are substantially different. These two fuels burn much faster, 3 to 8 times faster at standard conditions, than does propane.
Hydrogen and acetylene have another characteristic that differentiates them from fuels such as propane of butane. Under certain conditions, hydrogen and acetylene will detonate (explode) instead of deflagrate (burn). When a fuel detonates it releases all of its energy essentially instantaneously. The flame front speed in a detonation event is at hyper-Mach speeds (Mach 6 to 7), roughly 4,000 times faster than the laminar flame front speed (Mach ~0.001). Because of the very high burn rate in a detonation, the gun is subjected to a tremendous shock force. Most spudders believe that this level of stress is unsafe and that hydrogen and acetylene are unsafe fuels for a gun constructed from PVC.
Burnt Latke did a detailed study comparing the muzzle velocities of propane and MAPP. Using a 1.5"D riffled barrel shooting spuds, Latke found that MAPP out performed propane with muzzle velocities of 444 (+/-34) FPS for MAPP and 398 (+/-34) FPS for propane. So MAPP gave muzzle velocities that were about 12% faster than propane. This increase in performance is greater than what you would expect based solely on the "Heat per mole Oxygen" values for the two fuels. If the muzzle velocity scales as the square root of the ratio of the "Heat per mole Oxygen" values, then MAPP would be expected to give muzzle velocities about ~4% higher than propane. It is possible that the 12% increase Latke observed is not statistically different from the expected ~4% increase. Alternatively, MAPP may burn slightly faster than propane. Two of the components of MAPP, methyl acetylene and propadiene, would be expected to have burn speeds that are more similar to acetylene than propane.
There really isn't a "best fuel" for a combustion spud gun. If you want to get the maximum muzzle velocity from a gun the difference between the various fuels is relatively small. A typical combustion spud gun has so much shot to shot variation in velocity that the relatively small difference between fuels is not particularly relevant. Latke, as good of a spudder as there is, has done several studies using an accurate chronometer to measure muzzle velocities. In all of his studies with spuds as projectiles, using precisely metered fuels, the shot to shot variability in muzzle velocity is typically in the 10 to 20% range. In studies where Latke used the same round for several shots the shot to shot variability is still in the 5% range. So it really doesn't matter very much if one fuel has 5% more energy than another fuel.
The "best fuel" is the one that the gunner is most comfortable with, that is easiest to obtain and use, and is cheap. Beyond that there is really no significant difference between fuels.
There are other ways to get the best performance out of a combustion spudgun. Here is my list, from most important to least important;
Any of these four will give much greater increases in performance than will changing from, for example, propane to MAPP.
In 2013 a short paper was published in Eur. J. Phys. 34 (2013) 915-920 titled "Studying the Internal Ballistics of a Combustion Driven Potato Cannon using High-speed Video" by E D S Courtney and M W Courtney cited here and available for download here. In a nutshell, the authors report that acetylene is a 2.5x to 5x better fuel (based on muzzle velocity) than propane, ethanol, methanol and butane. They used high speed photography through a transparent barrel to measure the internal ballistics of spuds fired from a single gun fueled with the fuels listed above. Technically, most of the study was well done with one glaring exception. Though they accurately measured the amount of fuel for each shot it appears they completly negelected to calculate the proper amount of each fuel. Though the authors state "The amount of each experimental propellant was calculated to approximate a stoichometric mixture and considering the Upper Flammability Limit (UFL) and the Lower Flammability Limit (LFL), which in turn were affected by the volume of the combustion chamber." However, if you use stoichiometry then the UFL and LFL are irrelevant to the study. Indeed the volumes of fuels listed in the paper are not anywhere near stoichiometric mixtures. In the absense of data suggesting otherwise, it would be expected that the stoichiometirc amount of fuel would produce the best performance since it insures that all of the fuel and all of the oxidizer (oxygen in the air) is consumed. For example, the fuel volumes listed in the table for their gun with chamber volume of 1469.4 cubic centimeters (CC), for butane was 208 cc, corresponding to a fuel mixture of 16.5% volume/volume. (Volume/volume ratio is equivalent to the mole ratio.) For butane the fuel ratio was 12.7%. Both of the fuel raios are far from the stoichiometric values of 4.0 and 3.1% respectively. In addtion, those fuel percentages are above the normally quoted UFL for these two fuels (typicaly quoted as about 9% for propane and about 7% for butane). Overall, the fuels listed are expected to behave much more similarly than reported in this paper. The observed difference between the report and what is expected can be attributed to the grossly over-rich fuel mixtures used for all studied fuels.
Send me an email
5 Nov. 2017
©2008-2017 James Sluka