When fueling a combustion spudgun one question that occasionally
arises is; "How long does it take to equilibrate (fully mix) the fuel
with the air in the chamber?" To get the most oomph from a canon the
fuel and air in the chamber need to be well mixed. If most of the fuel
is at one end of the chamber the cannon's power will be reduced, indeed
it may not fire at all.
I've noticed that a cannon, without a chamber fan, and fueled with a
syringe is much more powerful on a warm summer day than it is on a cool
winter day. With a chamber fan the cannon's performance is much less
sensitive to the temperature. Perhaps the temperature dependence, in
the
absence of a fan, is due to slower mixing of the fuel and air on a cold
day.
It would be handy to have an estimate of how fast propane diffuses
(mixes) with air. To this end, I have constructed a simple combustion
chamber specifically designed to mix slowly. The chamber has a large
aspect ratio (length to width ratio), no chamber fan, and is fuel by
slow injection of propane near the end of the chamber opposite the
spark gap end.
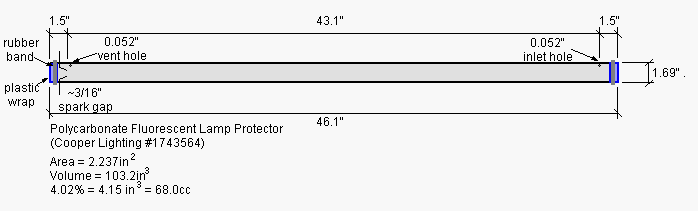
The test chamber was constructed from a 46" long by 1.69" diameter
(aspect ratio 27:1) polycarbonate fluorescent lamp
protector tube. These clear polycarbonate tubes are available at most
hardware
stores for a couple bucks each. The setup is shown below.

I closed the ends of the polycarbonate tube with plastic wrap
held in place with rubber bands (see photo below of the spark gap end
of the chamber). The chamber could be described as a "semi-open" tube;
at low pressures the chamber is well sealed, a modest rise in pressure
will blow the plastic wrap end caps off the chamber and limit the peak
pressure in the chamber.

The spark gap consists of a pair of "un-popped" pop rivets hot glued into holes in the tube. The ignition source was a "100KV" stun gun.
To make the small (0.05"D) filling vent hole visible I highlighted
it with a black marker (visible in the photo above).
The tests were carried out at temperatures that ranged from about 41F to 52F.
68cc of propane (4% of the chamber volume) was measured with a
syringe and injected over about 10 seconds into the small hole on the
end of the tube
opposite from the spark gap. The small vent hole on the spark end was
also open during injection. The holes were covered
with blue painter's tape after fueling. The blue tape survived repeated
firings
of the tube, without perforating or being dislodged, even though it was
peeled back and then reapplied for each refueling. The tube was held
horizontal
while fueling and was left to sit, without being disturbed, for the
times described below.
In the first test the chamber was fueled and the first ignition
attempt was 5 minutes after fueling. The chamber did not ignite. Four
more attempts were made at times up to 54 minutes without ignition. The
chamber then sat for an additional 38 minutes (92
minutes total) before another attempt was made at which time it
ignited, blowing both of the rubber banded plastic wrap end caps.
For the second test the chamber was purged with a fan, fueled as
before and ignition attempted at 19, 37, 47, 56, ... minutes up to a
last test at 6hrs after fueling. The chamber never ignited. This
suggests to me that there is some problem associated with multiple
unsuccessful attempts at ignition. It appears that unsuccessful
ignition attempts in some way consume the fuel and/or oxygen in the
chamber. Perhaps some of the fuel is burned even though the chamber
doesn't fully ignite. Alternatively, the spark converts some of the
oxygen in the chamber to ozone, which then reacts slowly with the
polycarbonate chamber or with some of the propane fuel. Repeated
nonproductive firings ends up reducing the fuel and/or oxygen level
below the lower combustion limit of propane in air.
To over come this problem I changed the testing procedure. In the
new method ignition is only attempted once per fueling cycle. If the
chamber does not ignite then it is purged and refueled and a new test
is started.
Tests using this new method successfully ignited after 111, 77, 62,
57, 49 and 42.5 minutes. The next attempt was at 31 minutes which
failed to ignite (the chamber also didn't ignite at 44 min but did
ignite at 176 minutes).
The next tests ignited at 37, 33 and 25 minutes.
Though I was trying to keep the refueling
method as consistent as possible there is still significant variability
in the mixing rate. There were ignition failures at 19 and 31 minutes
and success at 25 minutes. Given this variability, a precise
measurement of the diffusion rate is not possible. It is possible to
estimate an approximate diffusion rate. It appears that the time to
diffuse the ~44 inches between the inlet
port and the spark gap is between 25 and 31 minutes. Therefore, the
rate of diffusion is 1.4 to 1.8 inch/min. Note that
this time does not represent the time needed to fully equilibrate the
gases in the chamber. Instead, it is the time required to get a
combustible mixture at the spark gap. Since the lower flammability
limit of propane in air is about 3% the observed time to ignition may
represent the equivalent of two diffusional half-lifes. The first
half-life gets the propane concentration at the spark gap to 2% (50%
equilibration) which
won't ignite, the second half-life to 3% (75% equilibration) which will
ignite.
It appears that propane diffuses in air with a half-life rate of
0.28 to 0.35 minutes per inch at temperatures in the range of 41F to
52F. To get the fuel mixture to 97% of full equilibration will
require 5 half-lifes (calculated as [1 - 1/25]),
roughly 1.5
min/inch length between the fuel injection point and the spark
gap.
In the studies above I was trying to get the fuel to diffuse as
slowly as possible, the resulting diffusion rate is a worst case
scenario for this particular chamber. I then examined how much the
diffusion rate can be increased by simply injecting the fuel in a way
that will maximize mixing. The fastest that I can inject the 68cc fuel
from the syringe is about 1 second, roughly ten times faster than in
the previous studies. To see if a higher injection rate increases the
mixing rate, I did a series of firings using the higher fuel inject
rate. To reduce the back pressure in the system I added an additional
0.101" diameter vent hole to the spark gap end of the chamber in
addition to the original 0.052" hole. The chamber now has five times
greater vent area at the spark gap end.
The syringe needle is 1.5" long and has an OD of 0.049". I estimate
that the ID of the needle is about 0.043". Injecting 68cc (4.15in3)
of gas through a needle with an area of Pi(0.043/2)2 =
0.00145in2 in one second gives a gas velocity of;
4.15in3 / 0.00145in2 / 1sec = 239 FPS
As before, the fuel was injected, the holes covered with tape and,
after a waiting period, the chamber was ignited. Under these conditions
the chamber fired at 22, 19 and 14 minutes. The chamber didn't ignite
at 10 minutes (this loading also didn't ignite at 11 but did ignite at
14 minutes). The next test failed to ignite at 12 and 13 but did ignite
at 15 minutes. Assuming that only the first attempt at ignition is a
valid measurement, it appears the diffusion time to a combustible
mixture over the 44" distance is more than 12 and less than 14 minutes.
So, the t1/2 is about 0.15min/inch and 5t1/2 is
0.75 min/inch. Expressed as a diffusion velocity instead of a rate we
get 6.7inch/min.
Fast injection of the fuel increased the apparent diffusion rate by
a factor of two for this combustion chamber.
Table 1 (below) contains various physical and combustion properties for propane, methane and gasoline (Data from http://wps.com/LPG/WVU-review.html).
| Physical Properties | Propane | Methane | Gasoline |
| Specific Gravity at NTV (relative
to air) |
1.52 | 0.55 | 4.0 |
| Normal Boiling Point (K) | 231 | 111.5 | ~310-478 |
| Critical Pressure (atm) | 41.9 | 45.4 | 24.5-27 |
| Density of Liquid at NTP (kg / L) | 0.5077 | 0.4225 | ~0.70 |
| Density of Gas at NTP (kg / m3 ) | 1.96 | 0.6512 | ~4.40 |
| Density Ratio, NTP Liquid / NTP Gas |
259 | 649 | ~150 |
| Diffusion Coefficients in NTP air ( cm^2 / s ) | 0.10 | 0.16 | ~0.05 |
| Diffusion Velocity in NTP air ( cm / s ) | ~0.34 | ~0.51 | ~0.17 |
| Diffusion Velocity in NTP air ( inch / min ) | ~8.0 |
~12 |
~4.0 |
| Combustion Properties | Propane | Methane | Gasoline |
| Quenching Gap in TNP Air (mm) | 1.78 | 2.03 | 2.0 |
| Limits of Flammability in Vol, % | 2.1-10.4 | 5.3-15 | 1-7.6 |
| Limits of Detonation in Air Vol, % | 3.4-35 | 6.3-13.5 | 1.1-3.3 |
| Minimum Energy for Ignition in Air (mJ) | 0.305 | 0.29 | 0.24 |
| Auto ignition Temperature (K) | 740 | 813 | 501-744 |
| Flame Temperature in Air (K) | 2243 | 2148 | 2470 |
| Maximum Burning Velocity in NTP Air ( cm / s ) | 43-52 | 37-45 | 37-43 |
| Energy of Stoichiometric Mixture, ( MJ / mol ) | 3.79 | 3.58 | 3.91 |
The measured diffusion velocity of 6.7inch/min agrees reasonable
well with the value of ~8.0inch/min from Table 1.
Graham's
Law states that the rate of diffusion of a gas is inversely
proportional to the square root of the gas's molecular weight. We can
use this relationship to estimate how much faster, or slower, other
fuel gases would diffuse in this chamber.
| Fuel
Gas |
Molecular Weight (g/mol) |
Relative Diffusion Rate |
Slow Injection 5t1/2 (min/inch) |
| Butane |
58 |
0.9 |
1.7 |
| Propane |
44 |
1.00 |
1.5 |
| Ethane |
30 |
1.2 |
1.3 |
| Methane |
15 |
1.7 |
0.9 |
| Hydrogen |
2 |
4.7 |
0.3 |
This study was designed to measure the worst case diffusion rate.
In practice, there are several things that can be done to increase the
mixing rate of propane in air. A chamber fan should give
complete mixing in just a few seconds. Inverting the chamber slowly
several times should also significantly increase the diffusion rate as
will injecting the fuel at high velocity. Injecting the fuel at the
center of the chamber, instead of the end, will cut the distance the
fuel has to diffuse in half and give adequate mixing in half the time.